By Lori Allen, RPh, ISEAI Board Director, owner of Mixtures Pharmacy
We ask you to explain to the FDA why it is important for you to have continued access to compounded intranasal Vasoactive Intestinal Peptide (VIP).
The FDA is reviewing the status of VIP. They are accepting comments on regulations.gov through December 5th, 2019 and will then decide to:
- Have a hearing, or
- Make recently proposed rules a law and effectively remove VIP from our list of available products to compound
[Use this link to directly send your comments to the FDA before December 5th, 2019.]
You can copy the text below and paste it into the comments box:
To Whom it May Concern:
I currently use compounded intranasal VIP to help me (fill in why you use it – to function, not have brain fog, keep a job, spend time with my family, etc)
Without VIP, I feel (tell them how you feel – horrible, depressed, mentally foggy, unable to function, etc).
Without VIP my ability to function (let them know the impact of not having access to VIP – is severely limited, is marginal, would prevent me from holding a job, might put me on disability, etc)
(Add more of your story here)
Please reconsider keeping compounded intranasal VIP accessible to me, because of the positive impact it makes on my life.
Some history: The FDA’s Pharmacy Compounding Advisory Committee (PCAC) met back in 2016 and decided at that time there wasn’t enough information for them to move VIP from the Category 1 List, a list that was safe for the patient and available for compounding pharmacies to use, to the positive Bulk Substances List. On 9/15/19 the FDA released new rules (PDF).
About VIP
Vasoactive Intestinal Peptide (VIP) is considered a major regulatory peptide in the brains of mammals. It acts as a neurotransmitter or immunomodulator in not only the brain but in the heart, lung, pancreas, thyroid, genitourinary tract and the immune system.
VIP is made up from amino acids (28 to be exact). It belongs in the family of glucogon/secretin peptides which do a lot to control the hypothalamus-pituitary-adrenal (HPA) axis, and the endocrine system. While it was initially discovered in the small intestine, it is also produced throughout the body; in a tiny part of the hypothalamus called the suprachiasmatic nuclei (SCN, see image below), in the pancreas, as well as synthesized in the immune cells and the endocrine cells.
VIP’s actions are widespread and it is found in both the central and peripheral nervous systems.
Some of the more direct actions we see with VIP include stimulating contractility in the heart, causing vasodilation, increasing glycogenolysis, lowering arterial blood pressure and relaxing the smooth muscle of trachea, stomach and gall bladder.
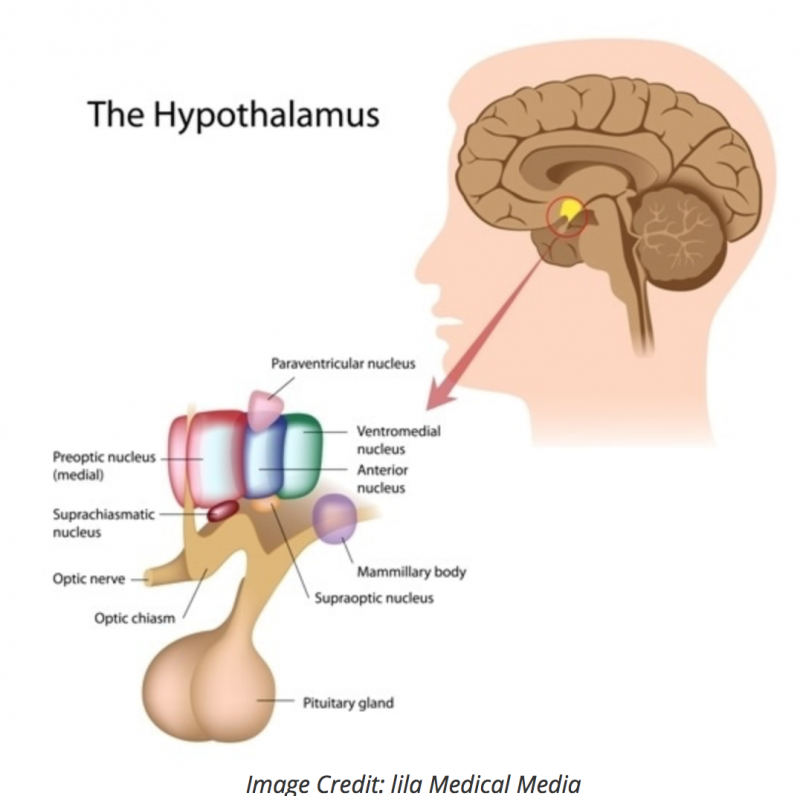
VIP works with the SCN to regulate the circadian system by synchronizing cues from light
Research on VIP
Research has identified the many diverse roles of VIP, including:
- A potent vasodilator and bronchodilator
- A coordinator of circadian rhythms in the brain
- A stimulator of insulin secretion
- A regulator of pro- and anti-inflammatory factor production
Due to its wide array of biological actions, VIP has been investigated for its therapeutic potential to treat a number of diseases such as asthma, arthritis, diabetes, and neurodegeneration. The FDA recognized VIP as a treatment for Pulmonary Hypertension (PH) and gave it orphan drug status for treatment of this fatal disease that typically give the patient 3 years from diagnosis. The diagnosis of PH is confirmed by VIP peptide deficiencies in the serum and lung tissue and confirmed with radioimmunoassay and immunohistochemistry.
Under normal conditions, VIP is present in the circulation at low levels (in serum, mean: approximately 42 pg/ml, normal range: 12.9 – 98.5 pg/ml). Circulating VIP is thought to originate from perivascular VIP-producing nerve fibers. Certain conditions, such as prolonged exercise, extended fasting, or gastrointestinal tumors (VIPoma), have been associated with elevated levels of circulating VIP. Although it is detected in blood, local VIP in tissues is primarily responsible for its biological effects.
VIP that is used for Pulmonary Hypertension is primarily given as an injection. There are current Phase 2 trials with on a 60 hour injection that is specifically designed to target the receptors in the lungs (PVAC2).
Most of you who are reading this are more familiar with the nasal spray delivery system. The studies show that VIP is found to cross the blood brain barrier (BBB) by a non-saturable transport system that doesn’t get interference from enzyme degradation and the amounts that end up in the brain are likely to affect brain function.
The findings also show that this transport of VIP is one way and VIP stays in the central nervous system (CNS) rather than being cleared through the blood. Further studies show that intranasal VIP produced higher levels of VIP in the brain when compared to intravenous administration.
Primary photo by Stage 7 Photography on Unsplash
References:
Aton SJ, Colwell CS, Harmar AJ, Waschek J and Herzog ED. 2005. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 8:476-483.
Bednarska, Olga, et al. “Vasoactive Intestinal Polypeptide and Mast Cells Regulate Increased Passage of Colonic Bacteria in Patients With Irritable Bowel Syndrome.” Gastroenterology, vol. 153, no. 4, 2017, doi:10.1053/j.gastro.2017.06.051.
Campos-Salinas, Jenny, et al. “Therapeutic Efficacy of Stable Analogues of Vasoactive Intestinal Peptide against Pathogens.” Journal of Biological Chemistry, vol. 289, no. 21, 2014, pp. 14583–14599., doi:10.1074/jbc.m114.560573.
Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D and Delgado M. 2005. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci USA 102:13562-13567.
Delgado M, Abad C, Martinez C, Laceta J and Gomariz RP. 2001. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med 7:563-568.
Delgado, Mario, et al. “Vasoactive Intestinal Peptide Protects against β-Amyloid-Induced Neurodegeneration by Inhibiting Microglia Activation at Multiple Levels.” Glia, vol. 56, no. 10, 2008, pp. 1091–1103., doi:10.1002/glia.20681.
Dogrukol-Ak, Dilek, et al. “Passage of VIP / PACAP / Secretin Family Across the Blood-Brain Barrier: Therapeutic Effects.” Current Pharmaceutical Design, vol. 10, no. 12, 2004, pp. 1325–1340., doi:10.2174/1381612043384934.
Dogrukol-Ak D, Banks WA, Tuncel N and Tuncel M. 2003. Passage of vasoactive intestinal peptide across the blood-brain barrier. Peptides 24:437-44.
Domschke, S, et al. “Vasoactive Intestinal Peptide in Man: Pharmacokinetics, Metabolic and Circulatory Effects.” Gut, vol. 19, no. 11, 1978, pp. 1049–1053., doi:10.1136/gut.19.11.1049.
Dufes, Christine, et al. “Brain Delivery of Vasoactive Intestinal Peptide (VIP) Following Nasal Administration to Rats.” International Journal of Pharmaceutics, vol. 255, no. 1-2, 2003, pp. 87–97., doi:10.1016/s0378-5173(03)00039-5.
Hejna M, Hamilton G, Brodowicz T, Haberl I, Fiebiger WC, Scheithauer W, Virgolini I, Köstler WJ, Oberhuber G and Raderer M. 2001. Serum levels of vasoactive intestinal peptide (VIP) in patients with adenocarcinomas of the gastrointestinal tract. Anticancer Res 21:1183-7.
Henning RJ and Sawmiller DR. 2001. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res 49:27-37.
Hilsted J, Galbo H, Sonne B, Schwartz T, Fahrenkrug J, de Muckadell OB, Lauritsen KB and Tronier B. 1980. Gastroenteropancreatic hormonal changes during exercise. Am J Physiol 239:G136-40.
Jayawardena, Dulari, et al. “Vasoactive Intestinal Peptide Nanomedicine for the Management of Inflammatory Bowel Disease.” Molecular Pharmaceutics, vol. 14, no. 11, 2017, pp. 3698–3708., doi:10.1021/acs.molpharmaceut.7b00452.
Jones, Jeff R., et al. “SCN VIP Neurons Are Essential for Normal Light-Mediated Resetting of the Circadian System.” The Journal of Neuroscience, vol. 38, no. 37, 2018, pp. 7986–7995., doi:10.1523/jneurosci.1322-18.2018.
Kulka M, Sheen CH, Tancowny BP, Grammer LC and Schleimer RP. 2008. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 123:398-410
“The Journal of Neurosci.” doi:10.1523/jneurosci.
Morell M, Souza-Moreira L and Gonzalez-Rey E. 2012. VIP in neurological diseases: more than a neuropeptide. Endocr Metab Immune Disord Drug Targets 12:323-332.
Neske, Garrett T., and Barry W. Connors. “Distinct Roles of SOM and VIP Interneurons during Cortical Up States.” Frontiers in Neural Circuits, vol. 10, 2016, doi:10.3389/fncir.2016.00052.
Nussdorfer, Gastone G, et al. “Secretin, Glucagon, Gastric Inhibitory Polypeptide, Parathyroid Hormone, and Related Peptides in the Regulation of the Hypothalamus– Pituitary–Adrenal Axis.” Peptides, vol. 21, no. 2, 2000, pp. 309–324., doi:10.1016/s0196-9781(99)00193-x.
Oktedalen O, Opstad PK, Waldum H and Jorde R. 1983. The fasting levels and the postprandial response of gastroenteropancreatic hormones before and after prolonged fasting. Scand J Gastroenterol18:555-60.
Palmer JB, Cuss FM and Barnes PJ. 1986a. VIP and PHM and their role in nonadrenergic inhibitory responses in isolated human airways. J Appl Physiol 61:1322-1328. Palmer JB, Cuss FM, Warren JB et al., 1986b. Effect of infused vasoactive intestinal peptide on airway function in normal subjects. Thorax 41:663-666.
Parker, Charles Thomas, et al. “Exemplar Abstract for Fluoribacter Bozemanae Garrity Et Al. 1980, Legionella Bozemanii (Sic) Brenner Et Al. 1980 pro Synon. Fluoribacter Bozemanae Garrity Et Al. 1980 and Legionella Bozemanae Corrig. Brenner Et Al. 1980 pro Synon. Fluoribacter Bozemanae Garrity Et Al. 1980.” The NamesforLife Abstracts, 2003, doi:10.1601/ex.2345.
Petkov, Ventzislav, et al. “Vasoactive Intestinal Peptide as a New Drug for Treatment of Primary Pulmonary Hypertension.” Journal of Clinical Investigation, vol. 111, no. 9, 2003, pp. 1339–1346., doi:10.1172/jci200317500.
Said, Sami I., and Viktor Mutt. “Potent Peripheral and Splanchnic Vasodilator Peptide from Normal Gut.” Nature, vol. 225, no. 5235, 1970, pp. 863–864., doi:10.1038/225863a0.
Sanlioglu AD, Karacay B, Balci MK, Griffith TS and Sanlioglu S. 2012. Therapeutic potential of VIP vs PACAP in diabetes. J Mol Endocrinol 49:R157-167.
Sawmiller, Darrell R, and Robert J. Henning. “Vasoactive Intestinal Peptide.” Handbook of Biologically Active Peptides, 2006, pp. 1215–1222., doi:10.1016/b978-012369442-3/50170-7.
Schebalin M, Said SI and Makhlouf GM. 1977. Stimulation of insulin and glucagon secretion by vasoactive intestinal peptide. Am J Physiol 232:E197-200.
Smalley, S. G. R., et al. “Immunomodulation of Innate Immune Responses by Vasoactive Intestinal Peptide (VIP): Its Therapeutic Potential in Inflammatory Disease.” Clinical & Experimental Immunology, vol. 157, no. 2, 2009, pp. 225–234., doi:10.1111/j.1365-2249.2009.03956.x.
Song, Min, et al. “VIP Enhances Phagocytosis of Fibrillar Beta-Amyloid by Microglia and Attenuates Amyloid Deposition in the Brain of APP/PS1 Mice.” PLoS ONE, vol. 7, no. 2, 2012, doi:10.1371/journal.pone.0029790.
“Vasoactive Intestinal Peptide.” Wikipedia, Wikimedia Foundation, 6 Sept. 2019, en.wikipedia.org/wiki/Vasoactive_intestinal_peptide.
Wernig, Karin, et al. “Depot Formulation of Vasoactive Intestinal Peptide by Protamine-Based Biodegradable Nanoparticles.” Journal of Controlled Release, vol. 130, no. 2, 2008, pp. 192–198., doi:10.1016/j.jconrel.2008.06.005.
White, Caitlin M., et al. “Therapeutic Potential of Vasoactive Intestinal Peptide and Its Receptors in Neurological Disorders.” CNS & Neurological Disorders – Drug Targets, vol. 9, no. 5, 2010, pp. 661–666., doi:10.2174/187152710793361595.
Wu, Dongmei, et al. “Prospect of Vasoactive Intestinal Peptide Therapy for COPD/PAH and Asthma: a Review.” Respiratory Research, vol. 12, no. 1, 2011, doi:10.1186/1465-9921-12-45.






VIP [Vasoactive Intestinal Peptide] has been documented to improve the quality of life enormously in desperately ill patients. We cannot afford to lose use of VIP! The U.S. Government FDA is holding hearings on why VIP should not be PROHIBITED for use. Doctor Shoemaker is appealing the FDA decision to prohibit the use of VIP. This appeal costs a lot of money. Please donate to Doctor Shoemaker’s Non-Profit to help him with the cost of the appeal.
https://www.facebook.com/donate/1133751103492243/?fundraiser_source=external_url
For the Fund Raiser to save VIP, you can also mail your donations to: If you prefer to mail a check, instead of donating online
Please Send Donations to:
CRBAI
500 Market St Suite 103.
Pocomoke MD 21851.
Make check payable to:
Center For Research on Biotoxin Associated illnesses Inc.
Thank you,
Ritchie C. Shoemaker, M.D.